1. We experimentally revealed the mechanism of interaction between multiphase heat/mass transport and reaction
kinetics in fuel cells and found the intrinsic relationship between the flow behaviour in the flow field and current.
This finding suggests that conventional models are incorrect because they make the assumption that the cell
power output is independent of flow patterns. In particular, we discovered the transient capillary blocking
phenomenon which occurs when the channel size becomes critically small and elucidated the bubble evolution
in the flow field due to electrochemical reactions. The understanding of how the flow behaviour and the electrochemical reaction led to an
analytical expression of the mass transfer coefficient that incorporates the effects of bubbles and channel ribs. This expression can be used to
determine the mass transfer coefficient by measuring the limiting current. The theory represents an effort in bridging the gap between multiphase
mass transport theory and electrochemistry.
A direct alcohol fuel cell
2. We experimentally demonstrated the intrinsic coupling between heat and mass transport in fuel cells. The fact that cell performance
increases proportionally with fuel concentration in direct methanol fuel cells (DMFC) is well known. Researchers have attributed the
better performance to an improved mass transport from the higher fuel concentration. We, however, found that the operating
temperature also increased with an increase in fuel concentration, which accelerates the kinetics of electrochemical reactions. This
discovery is important as it, for the first time, reveals the intrinsic coupling of heat and mass transport in fuel cells. The discovery not
only led to the invention of an innovative asymmetric electrode architecture, but also enabled us to understand the mechanism of
the coupled heat/mass transport and the kinetics of electrochemical reactions in fuel cells.
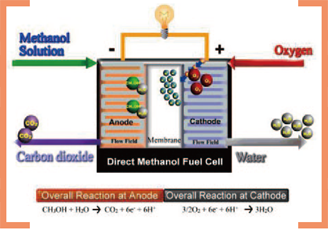
Working principle of a direct alcohol fuel cell
3. We developed a theoretical framework that describes the coupling of trans-scale,
multiphase heat/mass transport and electrochemical reactions. The framework was
developed on the basis of the newly gained understanding of coupled multiphase heat/mass transport and electrochemical reactions and the mechanism of improved
electrochemical kinetics with nano-electro-catalysts, proton/electron transport in the
nanoscale reaction layer, mass transport in the microscale diffusion layer and multiphase
flow in the macroscale flow field. In addition, it provides a solid foundation for the theory
of charge, heat and mass transport as well as the electrochemical reactions occurring in
fuel cells and extends the application of classical heat/mass transport theory.
Prof Tianshou ZHAO
Department of Mechanical and
Aerospace Engineering
The Hong Kong University of Science and
Technology
metzhao@ust.hk
