
Liver cancer is one of the most difficult cancers to treat. When the disease is diagnosed, patients often are in a critical condition with a life expectancy of only around six months. Surgery is the treatment of choice for only a small percentage of patients with localized disease. Alternative treatments may include chemotherapy, percutaneous ethanol injection etc. but these treatments have shown low response rates. For advanced liver cancer, there is no standard treatment. Liver cancer is also one of the most common cancers in the world. It is the second most common cancer in China with around 260,000 new cases being reported annually. The global rate rises to a million new cases a year while in Hong Kong the disease affects about 1,500 people every year. Proving just how deadly the disease can be, the death rate parallels the incidence rate – in Hong Kong more than 1,400 people die of the cancer every year while in the US where around 20,000 new cases are diagnosed annually, there are more than 18,000 deaths a year.
 |
| Test-tubes containing the anti-cancer drug solution |
Providing new hope for liver cancer sufferers, a new drug BCT-100 has been developed by a team of researchers at the Lo Ka Chung Centre for Natural Anti-Cancer Drug Development and Department of Applied Biology and Chemical Technology at The Hong Kong Polytechnic University. In a radical departure from more traditional cancer therapies, this drug seeks to inhibit the growth of cancer cells by starving them of nutrients essential for their survival. In this instance, the drug is based on recombinant human arginase, a natural human enzyme, which depletes an amino acid called arginine necessary for the growth of liver tumours.
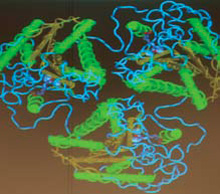 |
| A 3D model of the arginase molecule |
Dr Thomas Yun-chung Leung and Dr Thomas Wai-hung Lo, the two Principal Investigators of the project discussed these developments. Dr Leung said, "Knowledge of arginase and its effect on tumour cells has been around for some time and a couple of papers were published in the 1960s and 70s on the subject. However, subsequent studies were unable to prove the link and interest in the subject waned. In 2001, Dr Paul Cheng, an oncologist on ourteam had a patient with advanced liver |
|
 |
| Dr. Wai-Hung Lo and Dr. Yun-Chung Leung |
cancer who experienced a period of improvement in his condition. Out of curiosity, he tested the patient's blood levels for arginine and found them to be virtually undetectable. That was the spur for our research."
Arginine, necessary for cell survival, is produced by normal cells in the human body. Certain cancer cells however, such as liver cancer cells, are unable to produce arginine so they extract it instead from the blood. Depleting arginine in the blood thus literally starves the cancer cells to death while normal cells are unaffected as they can produce their own supply of the amino acid. Though the process sounds relatively simple a major stumbling block is the very short half-life of arginase, which is just a few hours. For it to be effective as a medication, it had to be both stabilized and its half-life prolonged to enable it to remove arginine in the blood over an extended period of time.
Dr Lo pointed out that the research team overcame this problem with a process called "pegylation" which attaches a polymer (polyethylene glycol or PEG) to the surface of the enzyme molecule, in essence protecting it from its surroundings. Subsequent tests on a number of different animals were successful and Phase I of clinical trials for the drug has also been successfully concluded. Optimum dosage levels have now been set and initial indications are that treatment of patients over 10 to 12 weeks should be sufficient. The trial has also shown the drug to be safe with minimal side effects. During trials on two terminally ill patients, one of them improved significantly. The drug also appeared to prolong his life – given just a month to live when he started the treatment he actually lived for several months longer.
Dr Leung concluded, "BCT-100 marks a major milestone both in Hong Kong and the field of cancer therapies. It is the first drug to be developed in Hong Kong to proceed to clinical trials and it also marks a cornerstone in the development of Hong Kong's biotechnology and pharmaceutical industries. The breakthrough also won us a Gold, as well as a Special Gold Award from the Ministry of Education and Research of Romania, at the 33rd International Exhibition of Inventions, New Techniques and Products in Geneva and has generated strong interest from around the world. Most importantly however is the ray of hope it will bring to liver cancer patients and possibly to other cancer patients as well."
Dr. Yun-Chung Leung
Department of Applied Biology and Chemical Technology,
The Hong Kong Polytechnic University
bctleung@inet.polyu.edu.hk
Dr. Wai-Hung Lo
Department of Applied Biology and Chemical Technology,
The Hong Kong Polytechnic University
bctlo@inet.polyu.edu.hk |
|