It is well known that a hot drink or a nourishing meal
is relaxing and helps to calm anxiety suggesting that
enhanced sensory vagal inputs originating from the gut modulate attitude and behavior. Although
vagal afferents are activated by noxious gastrointestinal
stimuli, the contribution of the vagal nerve to visceral pain remains unresolved.
Rodents do not have the forebrain structures to generate the cognitive
feelings of humans, the use of behavioral paradigms visceromotor
responses (VMR) to assess spinal pain reflexes in the conscious rat may
help to identify the regulatory role of the vagal nerve in visceral pain
sensation. We demonstrated that chronic subdiaphragmatic vagotomy
decreases the threshold and enhances the VMR to all grades of
colorectal distension (CRD) suggesting vagal nerves are involved in the
inhibitory modulation of visceral pain responses.
Vagus nerve stimulation with the neuro-cybernetic prosthesis generator
has been used clinically as a treatment for refractory epilepsy, major
depression, and gastric dysrhythmia. To date, the influences of
vagal nerve stimulation in visceral pain evoked by viscera nociceptive stimuli
have not been investigated. Vagal electrical stimulation by different
intensities may activate different types of nerve fibers. In this study,
vagal afferent neuronal responses to low or high intensity electrical
vagal stimulation (EVS) of afferent A or C fibers were distinguished
by calculating their conduction velocity. Here, we showed that CRD
produced contractions of the lateral abdominal musculature. High
intensity EVS (400 μA,) which activated C-type fibers had no effect on
CRD-induced abdominal pain. In contrast, low intensity electrical
vagal stimulation (40 μA) which activated vagal A-type fibers reduced
CRD-induced
abdominal muscle contractions. This response was not affected
by perivagal capsaicin-treatment. These observations
suggest that vagal afferent nerves modulate visceral
pain. Low intensity EVS which activates vagal afferent A- fibers
reduced visceral pain.
From left to right: Prof Ying Li, Miss Chun
Hao, Miss Ni Yan, Miss Jiahe Xu
|
|
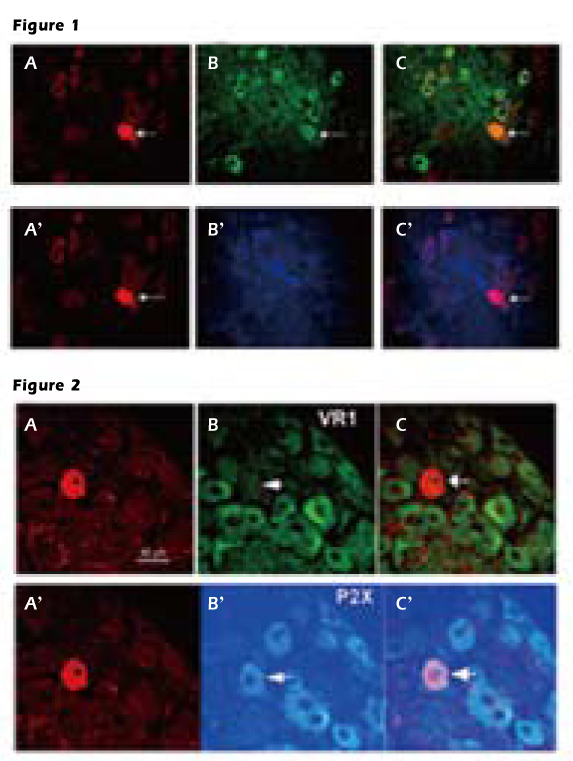
Figure 1. Vagal afferent C-type neuron
Upper panel A, the cell was marked by Neurobiotin. B, the ganglion contains
several neurons expressing VR1 recepor. C, the overlay of images shows that the
Neurobiotin-marked cell contains VR1 receptor. Lower panel A’, the same neuron
marked by Neurobiotin B’, incubated with P2X receptor antiserum C’, this Neurobiotin-labeled neuron does not express P2X receptor.
Figure 2. Vagal afferent A-type neuron
Upper panel A, the neuron labeled by Neurobioti B, the neuron contains VR1
receptors. C, overlay of images showing the Neurobiotin-marked cell does not
express VR1 receptor. Lower panel shows the same neurons marked by Neurobiotin contain P2X receptors.
To identify the neurotransmitters and receptors of vagal afferent
neurons activated by EVS single neuronal activities of nodose
neurons were recorded in vivo in rats, followed by juxtacellular
neurobiotin labeling. In consistent with our published data
intestinal perfusion of serotonin (5-HT) activates subpopulations of both A- and C-type vagal afferent neurons. Double
labeling immunocytochemistry showed that all of the C-type neurons
labeled by neurobiotin contained VR1 receptors (Fig. 1). In
contrast, while A-type neurons contained P2X receptors (Fig. 2), none of them
expressed VR1 receptors suggesting P2X and VR1 receptors on vagal
afferent neurons are involved in mediating distinct
5-HT activated autonomic functions.
In summary, we explore the visceral analgesic properties of
subdiaphragmatic vagus nerve in rats and show that acute low intensity
electrical vagal stimulation reduces visceral pain suggesting that a
group of vagal afferents innervating viscera may have remarkable functions
that are related to visceral pain inhibition. Thus, vagal nerve stimulation
may have therapeutic potential in visceral pain treatment.
Prof Ying LI
Department of Biology and Chemistry
City University of Hong Kong
yingli@cityu.edu.hk
|
